Viruses infect plants, animals, and humans. A virus’ spread from animals to humans could unleash pandemics like COVID-19 — significant public health crises with considerable economic and social fallout. To nip such infections in the bud, public health researchers have advocated the ‘One Health’ approach: monitoring and protecting plant, animal, environment, and human health in an integrated fashion.
Quick, easy, and cost-effective methods of detecting viral infections can go a long way in ensuring this outcome. Recently, researchers from Harvard University, Cambridge, and Jiangsu University, Zhenjiang, reported developing one such tool: it can detect if cells have been infected by a virus using only light and some knowledge of high-school physics.
Their paper was published in the journal Science Advances in March this year.
A fingerprint of infection
A viral infection can stress cells and change their shapes, sizes, and features. As the infection gains the upper hand and the body becomes ‘diseased’, the changes become more stark.
The researchers behind the new study translated these cellular changes into patterns that could be used to say if a cell had been infected. They infected cells from a pig’s testicles with pseudorabies virus, shone light on them through a microscope, and tracked how changes in the cells distorted the light.
The researchers recorded these distortions at different points of time so that the light data mimicked a progressing viral infection. Then they compared these distortions with those in light that had been shone through healthy cells. They finally reported that the difference between the two light patterns represented a ‘fingerprint’ of virus-infected cells.
Changes in contrast
The distortion in question referred to diffraction patterns. Diffraction is the tendency of light waves to spread out after they pass through narrow openings or around small objects. Once this diffracted light reaches, say, a wall, it renders a pattern of alternating light and dark rings or stripes around a dark centre.
The fingerprint was based on two parameters: the contrast between the light and dark stripes and the inverse differential moment, a mathematical value that defined how textured the diffraction pattern was.
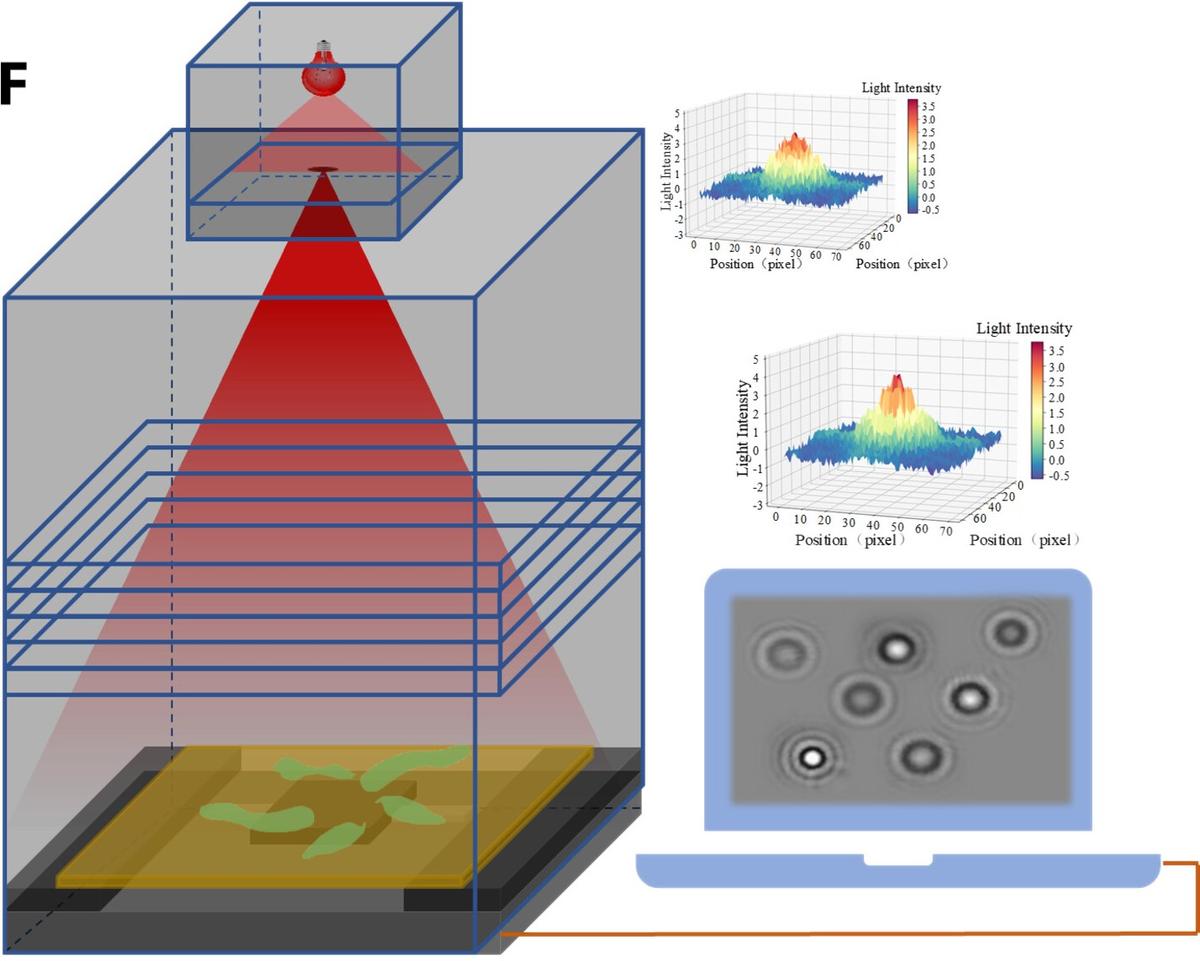
A schematic diagram of the test setup.
| Photo Credit:
DOI: 10.1126/sciadv.adl3466
The method can differentiate between uninfected, virus-infected, and dead cells. Virus-infected cells were elongated and had more clear boundaries than uninfected cells. This changed the contrast between light and dark stripes of the diffraction fingerprint, and increased the differences in light intensity.
Less time, money, complexity
Current methods to detect virus infections in cells are not straightforward. For example, in one technique, researchers isolate infected cells in the lab and add chemical reagents like dimethyl thiazolyl diphenyl tetrazolium bromide to them. The reagent destroys the cells, but not before the enzymes in the cells — called oxidoreductases and dehydrogenases — react with the reagent to produce purple crystals of a chemical entity called formazan. This colour change tells researchers the cells could have had a viral infection. Cells dying of a viral infection lack these enzymes and thus produce little to no amounts of formazan crystals.
The researchers compared their new technique with this standard for accuracy, time, and cost. They reported that their light-based method could detect viral infections as accurately or even more accurately than the standard method.
The new method was also cheaper than the standard: while the equipment cost for the standard method using chemical reagents is about $3,000 (Rs 2.5 lakh), the cost of the new method described in this paper was about a tenth. Many research facilities around the world also procure reagents from other places, adding potential time delays and vulnerability of their research to supply-chain inefficiencies.
Finally, the new method reportedly takes only about two hours to detect virus infected cells, against the 40 hours the current standard required.
Advantages for livestock
According to the paper, the researchers placed a sample of cells on slides under a microscope and light was shone on them. They obtained and subsequently analysed the diffraction fingerprint, and correlated each fingerprint with the corresponding condition of the cells. The team is yet to conduct real-world tests.
The low cost and ease of use point are likely to be lucrative to people working closely with animals, especially “livestock or common pets such as dogs and cats,” the researchers wrote in their paper. The new tool can help spot viral infections in their bodies as well as for “the selection and breeding of excellent livestock and poultry species at the cellular level”.
A new tool in the arsenal
Indeed, the new method could help catch viral infections early — which could be very helpful during, say, a virulent bird flu outbreak. The one going on around the world killed more than 131 million poultry in 81 countries in 2022 and 2023, according to the World Health Organisation.
Scientists typically test samples from any part of the bird the virus could infect: windpipe, cloaca (the waste chamber for urine and faeces), or their waste itself. If a bird dies after displaying the symptoms associated with the infection, they also look for the pathogen in the carcass’s tissues.
The methods they use include polymerase chain reaction (of the ‘PCR’ fame during the COVID-19 pandemic) or antigen tests, which detect the genes or proteins associated with the H5N1 virus. While the new method is not specific to certain kinds of viruses, it can help detect viral infections in general and help stakeholders take preventive measures in time to avoid significant losses.
In fact, the tool’s generic nature could also be an advantage by catching a viral infection that is not due to H5N1, perhaps even a new virus.

Against the spread of viruses
Viral outbreaks in animals have significant economic consequences. According to a 2018 study in the journal Transboundary and Emerging Diseases, bird flu outbreaks in the Kuttanad region of Kerala imposed losses of Rs 23 lakh in 2014 (2018 rate). The study also estimated the Government of Kerala spent Rs 5.4 crore “to contain the disease spread through massive culling, surveillance and monitoring of poultry and humans due to [the] zoonotic nature of the disease”.
Against this backdrop, a rapid and cost-effective way to detect viral infections could help improve surveillance and reduce the cost of selecting healthy animals or birds for breeding. The existing methods to select animals for breeding require expensive DNA-sequencing tools, even if these tools are very good at identifying some desired features in an animal.
The light-based tool could also help low- and middle income countries with limited resources to realise the WHO’s recommendation to “rapidly detect, report and respond to animal outbreaks as the first line of defence” against the spread of viruses.
Joel P. Joseph is a freelance science journalist and researcher.
